Which Describes Mendeleev's Use of the Term Eka-aluminum
Elements can be classified as metals nonmetals and ____. Be specific and methodical.
The Periodic Table Of The Elements
Was discovered four years after Mendeleev published his periodic table which included the predicted properties of eka-aluminum.
. Which elements and properties did bartleby. Aesthetic trippy drawings easy. Log in for more information.
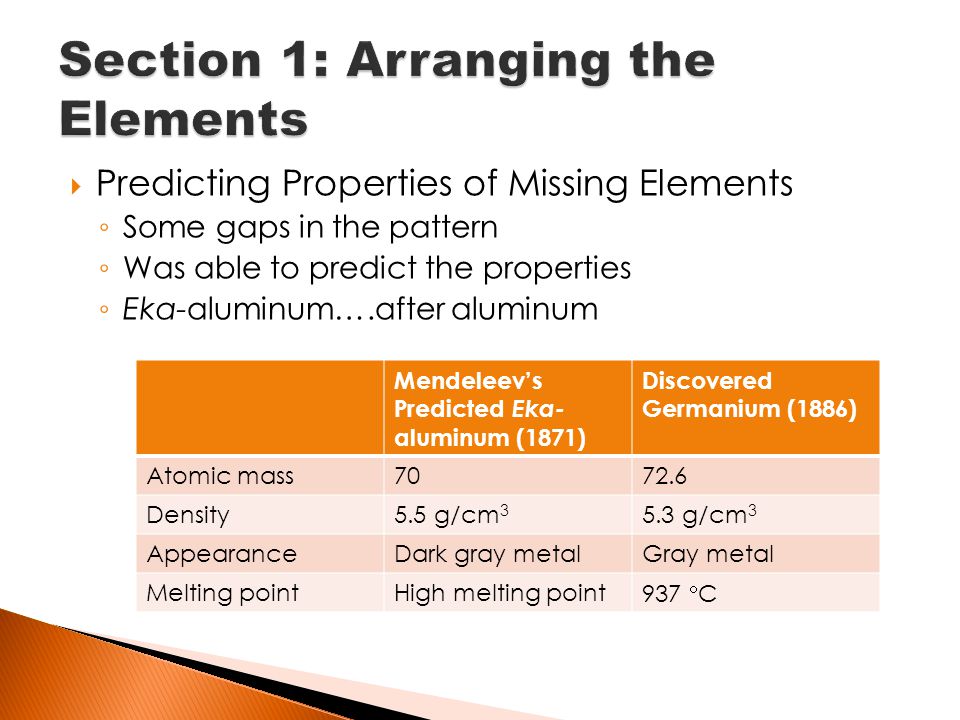
Unknown element he predicted would have properties similar to those of aluminum. Start studying MENDELEEVS PERIODIC TABLE CK12. What properties did Mendeleev predict that eka-aluminum would have-it would be a soft metal -it would have a low melting point -it would have a density of 59 gcm3.
Mendeleev names unnamed elements as EKA- Boron EKA- Aluminium and EKA Silicon which were later replaced as Scandium Gallium and germanium respectively. He named one of the element as eka-aluminium which was later discovered as Gallium. Mendeleevs periodic table was useful because it enabled scientists to predict properties of unknown _____ elements.
Describe how Mendeleev organized the elements into rows and columns in is period table He arranged the elements into rows in order of increasing mass so that elements with similar properties were in the same column. What was mendeleevs name for galliumhow to refer a friend in capgemini. B i Eka boron.
To give provisional names to his predicted elements Mendeleev used the prefixes eka- ˈ iː k ə- dvi- or dwi- and tri- from the Sanskrit names of digits 1 2 and 3 depending upon whether the predicted element was one two or three places down from the known element of the same group in his table. Rare isotope of aluminum. Eka boron is The element Scandium.
Which describes mendeleevs use of the term ekaaluminum. Which describes mendeleevs use of the term ekaaluminum. Use the beryllium magnesium and.
A Eka-aluminium and gallium are the two names of the same element as Eka-Aluminum has almost exactly the same properties as the actual properties of the gallium element. Which best describes the trends in electronegativity on the periodic table. Describe the features of dobereiners triads.
Does nsa still collect data 2020 Under. Eka-Aluminium Eka-boron were discovered by Moseley. For example germanium was called eka-silicon until its discovery in 1886.
Mendeleev used the word eka aluminium for gallium since it was not discovered at that time. Rare isotope of aluminum. Which elements and properties did Mendeleev examine when he predicted the properties of eka-aluminum and eka-silicon.
The periodic table is a tabular arrangement of the elements according to their atomic masses. Mendeleev predicted the properties of some of the elements during the classification which were not yet discovered. Were Mendeleevs predictions close to the actual properties of new elements.
Correct answer to the question How do you think your life might be different if you lived under a dictatorship instead of democracy. Mendeleev used the word eka aluminium for gallium since it was not discovered at that time. Learn vocabulary terms and more with flashcards games and other study tools.
Atomic mass density melting point formula of chloride and formula of oxide are almost the same. Eka silicon predicted by Mendeleev is Germanium. False Mendeleev Mendelevium atomic number 101 was synthesized in the year 1955 and commemorates Mendeleevs contributions to modern chemistry.
This will help you with completing this lab. Mendeleev gave the name eka-aluminum to a an A. Mixture of aluminum and an unknown element.
April 11 2022 Posted By. Mendeleev predicted that the undiscovered element he called eka-aluminum would have a ____ melting point.

Comments
Post a Comment