Which of the Following Would Form an Electrolyte Solution Apex
These substances constitute an important class of compounds called electrolytesSubstances that do not yield ions when dissolved are called nonelectrolytesIf the physical or chemical process that generates the ions is essentially 100. By this formula m MWIt 96500n.
What Is The Difference Between Electrolytes And Non Electrolytes Quora
The physical state of a solution is always the same as the sisolved solute.
. Check all that apply. When an aqueous solution is subjected to electrolysis the oxidation or reduction of water can be a competing process and may dominate if the applied voltage is sufficiently great. ExplanationA solution is a homogeneous mixture of two or more substances.
Which of the following are defining traits of solution. What is the hydrogen ion concentration of an aqueous solution have a pH of 472. How would an energy graph for this reaction look.
D all of the above have the same degree of basicity. B electrical energy chemical energy. When some substances are dissolved in water they undergo either a physical or a chemical change that yields ions in solution.
Which of the following would form an electrolyte solution. Strong electrolyte strong acid Identify NaCl. A disolved solute will not settle out or separate from the solvent in a solution.
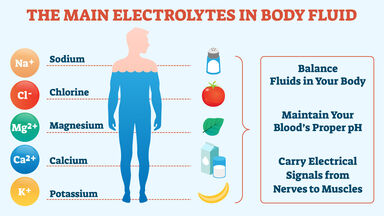
Learn vocabulary terms and more with flashcards games and other study tools. In an electrochemical cell the overall redox reaction is composed of two half-reactions. Gatorade as an electrolyte solution The sports drink Gatorade advertises that it contains electrolytes because it contains sodium potassium magnesium and other ions.
Electrical energy 2 H 2 O 2 2 H 2 O 2. Some examples of a non-electrolyte solution in water would be a solution of sugar or a solution of urea. T 47818 seconds 797 minutes.
A solution has a constant compostition throughout. When some substances are dissolved in water they undergo either a physical or a chemical change that yields ions in solution. D all of the above have the same degree of basicity.
C a solution whose OH- 10-14. 875 65454t 965002. These substances constitute an important class of compounds called electrolytesSubstances that do not yield ions when dissolved are called nonelectrolytesIf the physical or chemical process that generates the ions is essentially 100.
C Oxidation at anode ve charged electrode Reduction at cathode ve charged electrode. When humans sweat we lose ions necessary for vital bodily functions. When some substances are dissolved in water they undergo either a physical or a chemical change that yields ions in solution.
Ionic transport in the greater part of the electrolyte is by ordinary thermal diffusion the statistical tendency of concentrations to become uniform. In the human body electrolytes have. The energy of methane and oxygen would be higher than the energy of carbon.
To replenish them we need to consume more ions often in the form of an electrolyte solution. B a solution whose H 100. Weak electrolyte weak acid Identify sugar.
These substances constitute an important class of compounds called electrolytesSubstances that do not yield ions when dissolved are called nonelectrolytesIf the physical or chemical process that generates the ions is essentially 100. The oxidation takes place at the anode and the other half-reaction ie. A solution must have a solute a substance that can be dissolved or a soluble substance and a solvent a substance mostly liquid that can dissolve another substance For a substance to be dissolved it depends on its degree of solubility.
Would form would form a non-electrolyte solution in water. What precipitate is most likely formed from a solution containing Ba2 Na1 OH-1 and CO3-2. A solution can only occur in water B.
Correct option is A For electrolytic cell following are true statements. Start studying Chemistry APEX. During the combustion of methane CH4 with oxygen O2 energy is released as carbon dioxide CO2 and water H2O form.
Examples Of Electrolytes Basic Explanation And Purpose
What Is The Difference Between Electrolytes And Non Electrolytes Quora

Which Of The Following Would Form An Electrolyte Solution O A Oil In Water O B Sand In Water C Brainly Com
Comments
Post a Comment